Poster Presentation 23rd Annual Lorne Proteomics Symposium 2018
Carcinoembryonic antigen glycosylation – a highly underestimated cancer marker? (#110)
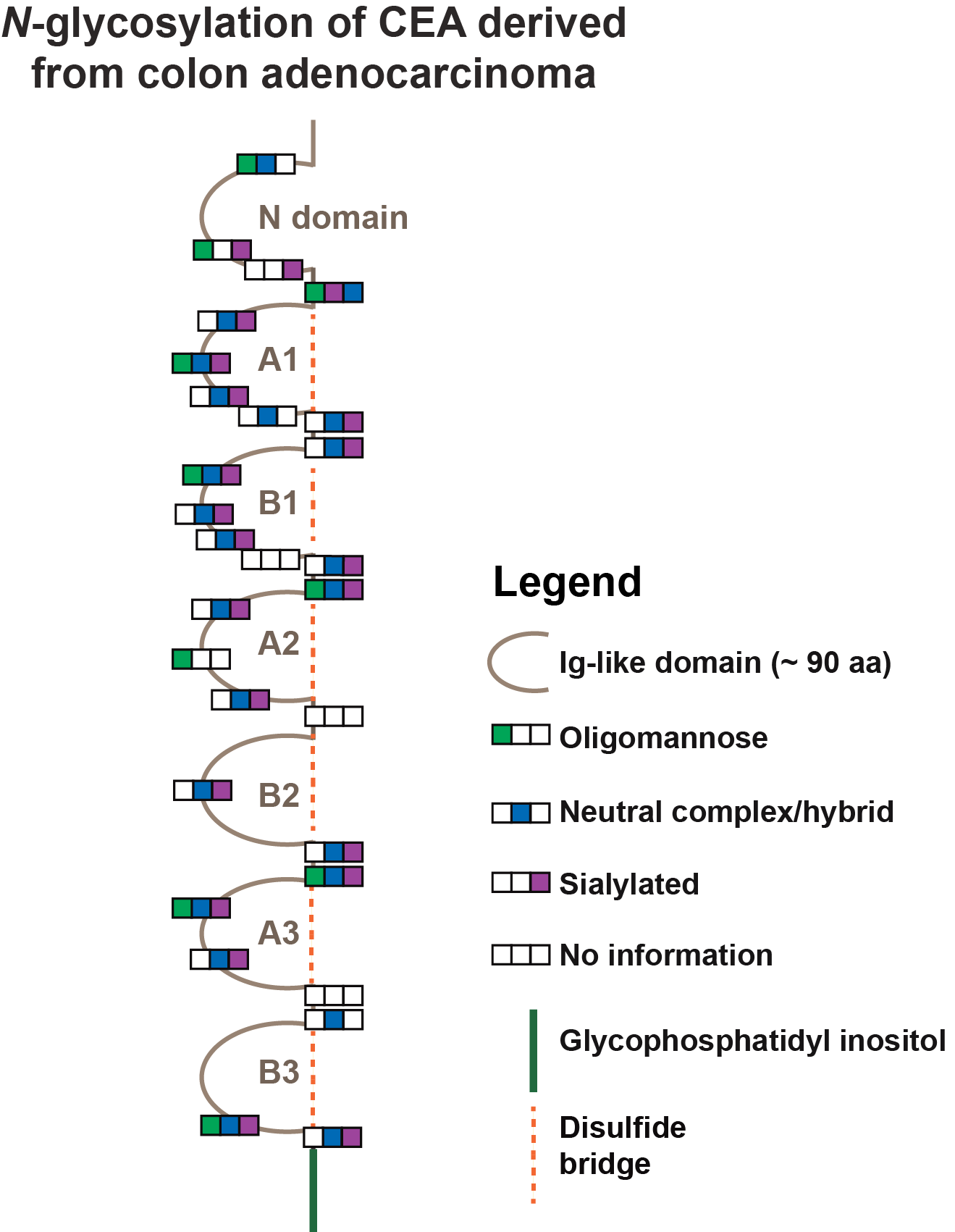 Carcinoembryonic antigen (CEA) is a widely used tumour marker for a variety of different cancers. With 28 potential N-glycosylation sites distributed over its seven Ig-like domains CEA is also a highly glycosylated protein. However, to date information on CEA specific glycosylation in health and disease is scarce. To close this gap and evaluate the diagnostic/prognostic potential embedded in CEA glycosylation we used highly sensitive and selective glycomics & glycoproteomics technologies to uncover CEA glycosylation signatures.
Carcinoembryonic antigen (CEA) is a widely used tumour marker for a variety of different cancers. With 28 potential N-glycosylation sites distributed over its seven Ig-like domains CEA is also a highly glycosylated protein. However, to date information on CEA specific glycosylation in health and disease is scarce. To close this gap and evaluate the diagnostic/prognostic potential embedded in CEA glycosylation we used highly sensitive and selective glycomics & glycoproteomics technologies to uncover CEA glycosylation signatures.
Purified CEA was obtained from human colon adenocarcinoma (cell line and tissue), liver metastasis of colon adenocarcinoma and ascites fluid. Glycopeptides obtained by pronase digestion were analysed in a dual LC-setup using reversed phase and porous-graphitized-carbon (PGC) nano-LC-ESI-MS/MS within a single run, while independent PGC nano-LC-ESI-MS/MS glycomics provided a hitherto unprecedented in-depth view on CEA glycosylation.
Glycoproteomics identified 28 N-glycosylation sites exhibiting varying occupancy rates of oligomannose, neutral and sialylated N-glycans (site-specific glycosylation). These occupancy rates also differed between CEAs from different origins. In all CEAs, however, site Asn309 was found to be not glycosylated. Unexpectedly, in the functionally important N-terminal domain Asn76 was identified to be glycosylated within a non-canonical sequence motif NRQ. CEA has been found to be one of most diversely N-glycosylated proteins described to date carrying over 250 different N-glycan structures. We identified distinct source dependent glycosylation features in N-glycan branching, degree of sialylation and level of bisecting N-glycans, indicating that CEA glycosylation provides excellent opportunities to improve currently existing CEA based cancer diagnostics. CEA was also found carrying the sialyl-Lewis X epitope, which is an important Lewis blood group antigen reportedly involved in cell-cell recognition and metastasis. A better understanding of CEA glycosylation signatures will also open novel avenues to study CEA's involvement in cancer progression and metastasis.